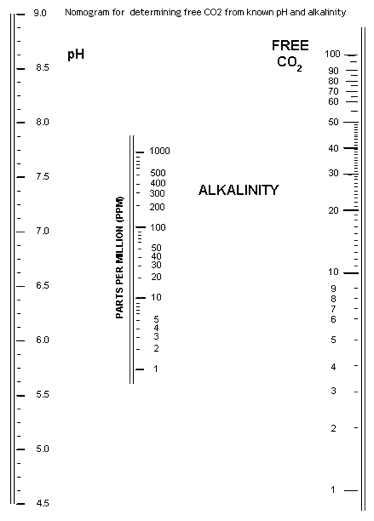
Carbon dioxide
This nomogram is useful for looking at free CO2 levels in water, based on pH and alkalinity

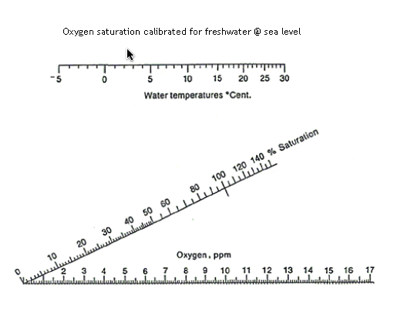
Oxygen
Like the carbon dioxide nomogram this one is useful for quickie conversions and estimates of oxygen content and saturation at different temperatures

Section 30 health checks
Before fish can be consented for introduction into a mandatory site (where water can flow from one body of water to another, or is in the floodplain) under Section 30, a sample of those fish must pass a mandatory health check. The fish examined for these health checks must be representative of the fish to be stocked. This means that there are rules for the number, size and species of fish to be examined for a health check.
The rules are based on evidence that:
• we need to examine a certain number of fish to have an acceptable chance of detecting an infection of Category 2 or novel parasites;
• some parasites are specific to the species of fish they will infect;
• some parasites are specific to the size of fish they prefer to infect.
Currently we only offer this service for salmonid producers having annual checks. We require the fish brought live to our laboratory, the test takes twice as long (and so costs more) when done on site, the EA require the test to start within 15 minutes of death of the fish.
For each site to be checked
• 30 fish sample
• Minimum of 10 per species to be moved, 15 rainbow trout + 15 brown trout
of these, then
• Trout are considered a ‘small species’ and so in addition to fry (<5cm fork length) two size ranges are recognised - above and below 15cm fork length. For the size of fish to be moved a minimum of 5 fish must be checked , so 7 fish can be < 15cm and 8 > 15cm of which a minimum of 5 must be of stock size
The lab setup and some common parasites looked for during the check are shown in the parasite gallery
The rules are based on evidence that:
• we need to examine a certain number of fish to have an acceptable chance of detecting an infection of Category 2 or novel parasites;
• some parasites are specific to the species of fish they will infect;
• some parasites are specific to the size of fish they prefer to infect.
Currently we only offer this service for salmonid producers having annual checks. We require the fish brought live to our laboratory, the test takes twice as long (and so costs more) when done on site, the EA require the test to start within 15 minutes of death of the fish.
For each site to be checked
• 30 fish sample
• Minimum of 10 per species to be moved, 15 rainbow trout + 15 brown trout
of these, then
• Trout are considered a ‘small species’ and so in addition to fry (<5cm fork length) two size ranges are recognised - above and below 15cm fork length. For the size of fish to be moved a minimum of 5 fish must be checked , so 7 fish can be < 15cm and 8 > 15cm of which a minimum of 5 must be of stock size
The lab setup and some common parasites looked for during the check are shown in the parasite gallery
SIC & STC
There are mechanisms in place with the Veterinary Medicines Directorate (VMD) to make available products which are licensed in the rest of Europe or the rest of the world. A Special Import Certificate (SIC) is used to justify bringing drugs in from Europe, whilst a Special Treatment Certificate (STC) is used for the rest of the world. Details on manufacture, licensing, quality control and strong evidence of need - usually based on animal welfare are needed. Obtaining these takes some time, especially if the product of interest has never been imported before. Information has to be gathered, the form filled in and submitted to VMD.

